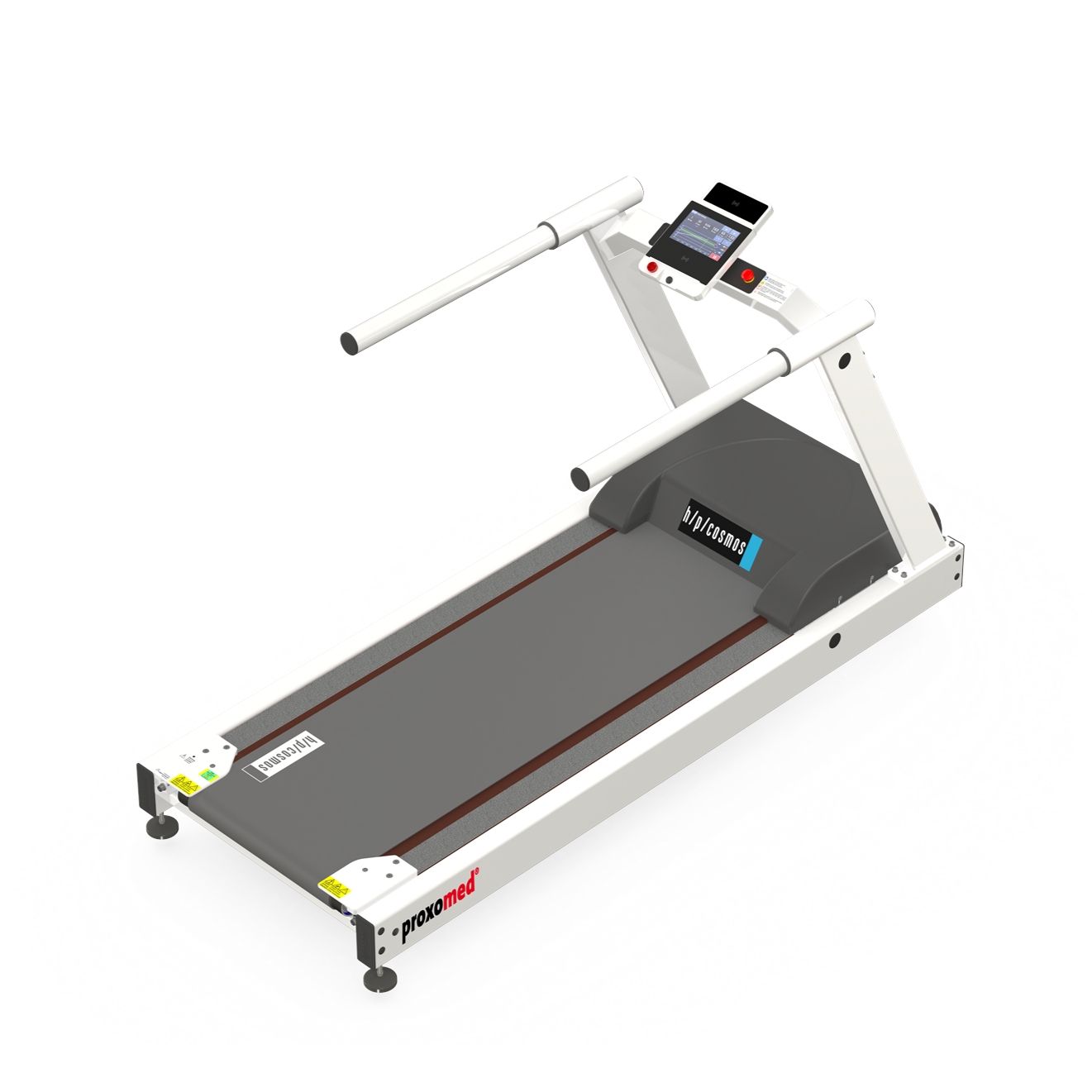
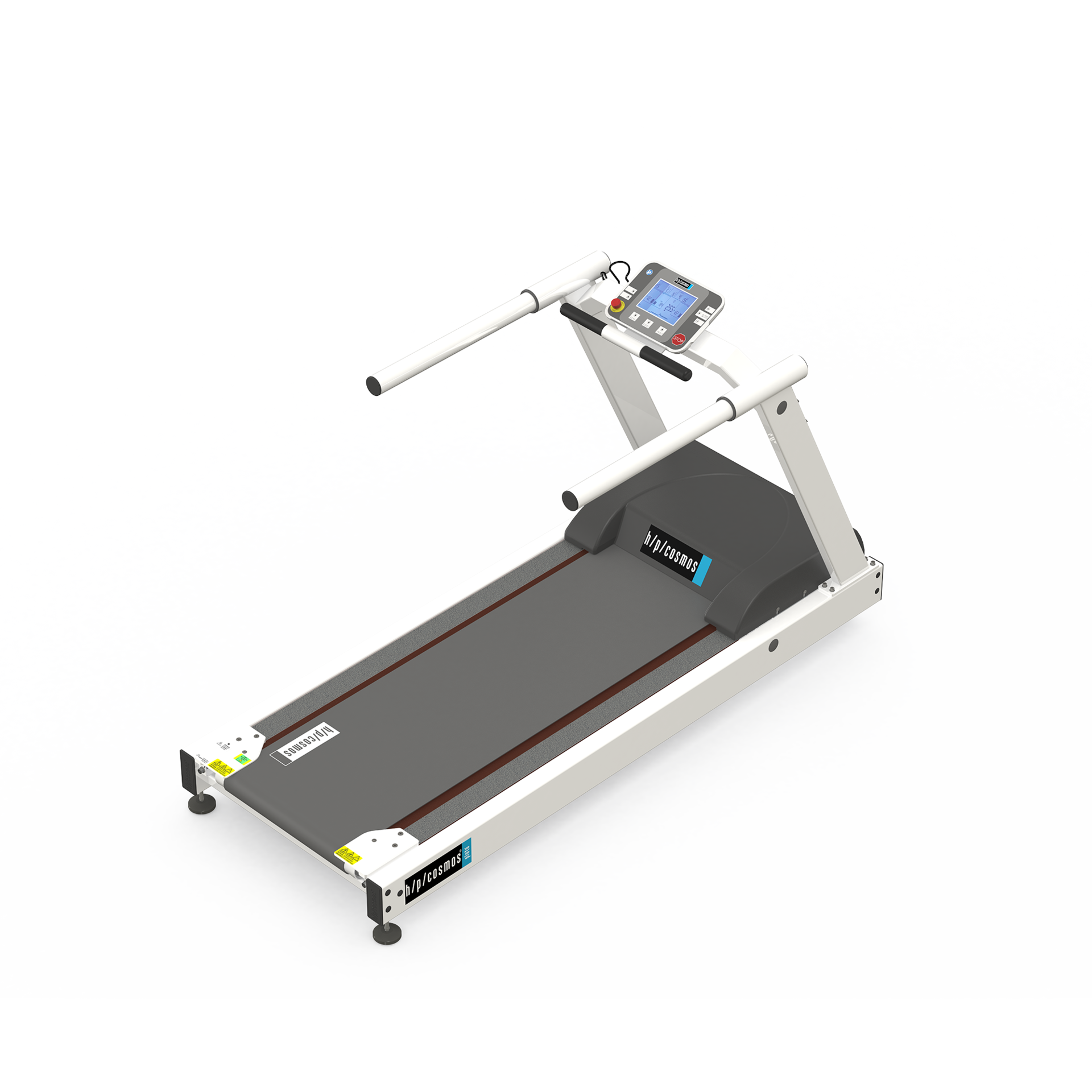
From health and fitness-related sports to medical endurance training, kardiomed 540 products offer diversity of application depending on the physical capacity and training goals of your patients, and thus high investment security.
Made in Germany - the complete series of units is manufactured in Germany. This secures jobs, saves resources - and thus the environment - and gives enormous flexibility in order-related production.
Independence from the power grid
The mains-independent/cable-free operation allows free choice of unit positioning in your facility, which can be changed quickly and conveniently if required.
A special feature is the monitor with full-colour TFT touch, which can be operated independently of the mains even at low speeds and power levels.
The brake, driven by its own movement, works with absolute precision. The unique control electronics make it possible to safely guarantee medical accuracy requirements over a power range of 15-1000 watts (depending on the unit). The electricity generated is first used to power the monitor. Any surplus generated charges a capacitor so that subsequent users can enter their training parameters without pedalling.
7-inch capacitive touch display
The capacitive 7-inch touch display is intuitive to use and features a variety of appealing views and enhanced displays.
For the basic cycle, comfort cycle and cross walk, the motion balance light system now also displays the force distribution between the sides of the body as standard.
The optional "program package" extends the standard training programmes e.g. with isokinetic training, intervals and serious games for certain types of equipment. This package also includes the full range of functions of the worldwide patented motion balance system.
The motion balance system was developed to identify and intelligibly display performance differences between the left and right extremities of the body.
The aim is to identify imbalances and reduce them through targeted training. Furthermore, the training programs and games should improve or restore the neuronal control of the affected muscles (e.g. after a stroke). Most users need an incentive to train specifically over a longer period of time. This is ensured by specially developed training programs called "serious games", i.e. games with a highly challenging character and direct feedback to the user.
Through ongoing testing, the motion balance system can be used to monitor and document development following a training intervention.
Three special games
The motion balance system includes three special games that promote targeted control of leg activities while training strength and endurance. Result indices allow evaluation of the training success by means of a simple parameter.
Slalom game
The goals appear alternately on the left and right sides of the screen. This means that the legs are also alternated. Stepping harder on the left side makes the skis move to the right; stepping harder on the right side makes the skis move to the left.
If the trainee has successfully moved around a gate, the backlight of the screen turns green and the task is considered successful. If he fails to do so or drives into the gate, the backlight turns red. The gates must be driven around on the short side.
Parameters:
- Number of goals
- Difficulty (=goal distance)
- Initial torque
Balance game
One after the other, weights of different sizes fall on the left or right pan. The imbalance must be compensated for by pedaling more forcefully (not faster). The balance must be maintained for a period of between 1 and 5 seconds (depending on the difficulty level set).
When the balance is reached, the backlight changes to green. If it is held for the duration of 1 to 5 seconds, this task is evaluated as successful, failed attempts are visualized in red. This is followed by the next weight/task.
Parameters:
- Difficulty (=duration of the compensation)
- Number of attempts
- Initial torque
Freeway game
The Freeway/Motorway game is the optimal introduction for exercisers who have just started playing the games or who have a significant imbalance in the body extremities, for example, due to a medical condition.
The difficulty level to be selected at the beginning determines the sensitivity of the car to be moved. For example, a lower difficulty level tolerates more uneven pedaling. The goal of the game is to keep your car in the center of the road. A successfully passed obstacle is confirmed with a green flash of the backlight.
Parameters:
- Difficulty (=sensitivity with which the game reacts to imbalance)
- Number of obstacles
- Initial torque
kardiomed 540 products